Study with Quizlet and memorize flashcards containing terms like The atmospheric pressure in Denver, CO is 633 mmHg. What is this pressure in atm?, A gas sample in a closed, expandable container of initial volume 5.00 L was allowed to warm from 25°C to 35°C. What was its new volume?, A gas sample contains 4.0 g of CH₄ and 2.0g of He. What is the volume of the sample at STP? and more.
Why does air pressure change so much from area to area? What is air pressure? Are there places where air pressure is healthier or less healthy? If one is in water, is
Chemistry questions and answers. Question 31 The atmospheric pressure in Denver, CO is 633 mmHg. What is this pressure in atm? O A 1.20 atm B 633 atm O C 0.833 atm O D 1.00 atm Unanswered Fill-in–the-blank questions: Complete the following sentences with the correct answer for each blank. Question 32 Fill in the Blanks As the temperature of a

Source Image: pearson.com
Download Image
The atmospheric pressure in Denver, CO is 633 mmHg. What is this pressure in atm? * .833 atm 633mmHg / 760atm = .832947 0.100 mole of lithium weighs * 0.6494 g Li = 6.94 x .100 = .6494 In any chemical reaction, the rate of the reaction can be increased by

Source Image: quizlet.com
Download Image
SOLVED: The atmospheric pressure in Denver, CO is 633 mmHg. What is this pressure in atm? VIDEO ANSWER: We are asked to calculate the pressure value in terms of 8. 760 m of h g is equal to 180 m. From here. The unit…
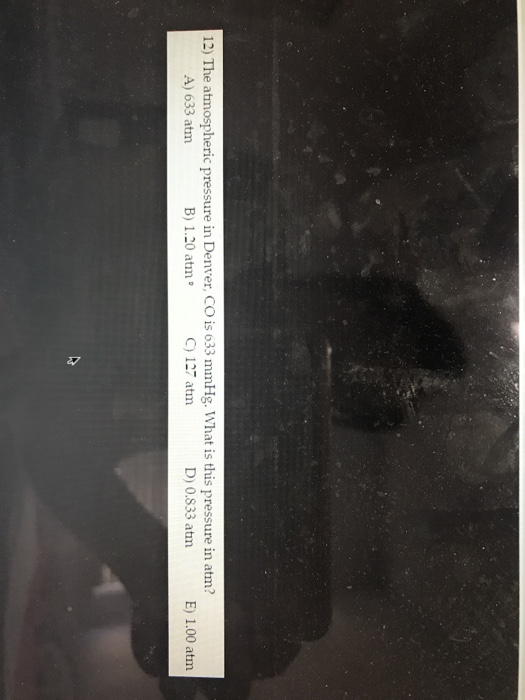
Source Image: chegg.com
Download Image
The Atmospheric Pressure In Denver Co Is 633 Mm Hg
VIDEO ANSWER: We are asked to calculate the pressure value in terms of 8. 760 m of h g is equal to 180 m. From here. The unit… The atmospheric pressure in Denver, CO is 633 mmHg. What is this pressure in atm? 0.833 atm 127 atm 633 atm 1.00 atm 1.20 atm. BUY. Chemistry: Matter and Change.
Solved 12) The atmospheric pressure in Denver, CO is 633 | Chegg.com
6 people found it helpful. skyluke89. 1 atm of pressure corresponds to 760 mmHg. Therefore, we can set a simple proportion to find how many atmospheres of pressure correspond to 633 mmHg, in the following way: And if we solve this proportion, we find the pressure in atmospheres: arrow right. Explore similar answers. The pressure in Denver, Colorado (elevation 5280 ft), averages a… | Channels for Pearson+

Source Image: pearson.com
Download Image
The pressure in Denver, Colorado averages about 632 mm hg. How many atmospheres is this? – Quora 6 people found it helpful. skyluke89. 1 atm of pressure corresponds to 760 mmHg. Therefore, we can set a simple proportion to find how many atmospheres of pressure correspond to 633 mmHg, in the following way: And if we solve this proportion, we find the pressure in atmospheres: arrow right. Explore similar answers.
Source Image: quora.com
Download Image
Why does air pressure change so much from area to area? What is air pressure? Are there places where air pressure is healthier or less healthy? If one is in water, is Study with Quizlet and memorize flashcards containing terms like The atmospheric pressure in Denver, CO is 633 mmHg. What is this pressure in atm?, A gas sample in a closed, expandable container of initial volume 5.00 L was allowed to warm from 25°C to 35°C. What was its new volume?, A gas sample contains 4.0 g of CH₄ and 2.0g of He. What is the volume of the sample at STP? and more.
Source Image: quora.com
Download Image
SOLVED: The atmospheric pressure in Denver, CO is 633 mmHg. What is this pressure in atm? The atmospheric pressure in Denver, CO is 633 mmHg. What is this pressure in atm? * .833 atm 633mmHg / 760atm = .832947 0.100 mole of lithium weighs * 0.6494 g Li = 6.94 x .100 = .6494 In any chemical reaction, the rate of the reaction can be increased by

Source Image: numerade.com
Download Image
SOLVED: If atmospheric pressure is 1.013 x 10^5 N/m^2 and creates a column of mercury 760 mm high. What is the density of mercury in kg/m^3 ? Give answer in standard form( If the atmospheric pressure is 800 mmHg, then what is the pressure of the dry gas you collected? When 0.600 liters Ar at 1.20 atm and 227 C is mixed with 0.200 liters of O2 at 501 torr and 127 C in a 400 ml flask at 27 C, what is the pressure in the flask?
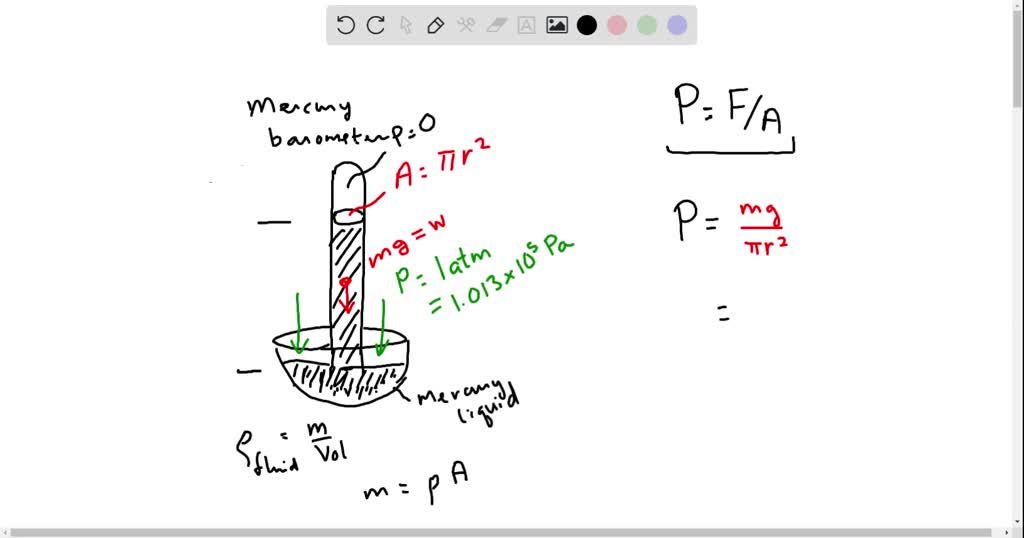
Source Image: numerade.com
Download Image
⏩SOLVED:Make the indicated pressure conversions. a. 45.2 kPa to… | Numerade VIDEO ANSWER: We are asked to calculate the pressure value in terms of 8. 760 m of h g is equal to 180 m. From here. The unit…
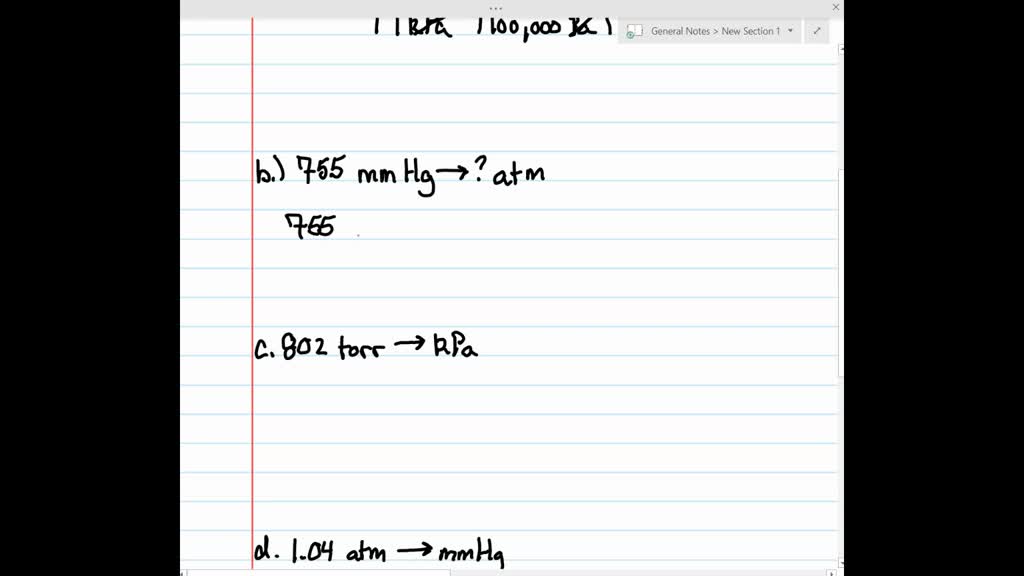
Source Image: numerade.com
Download Image
Acid-Base Homeostasis at the High Altitude | SpringerLink The atmospheric pressure in Denver, CO is 633 mmHg. What is this pressure in atm? 0.833 atm 127 atm 633 atm 1.00 atm 1.20 atm. BUY. Chemistry: Matter and Change.

Source Image: link.springer.com
Download Image
The pressure in Denver, Colorado averages about 632 mm hg. How many atmospheres is this? – Quora
Acid-Base Homeostasis at the High Altitude | SpringerLink Chemistry questions and answers. Question 31 The atmospheric pressure in Denver, CO is 633 mmHg. What is this pressure in atm? O A 1.20 atm B 633 atm O C 0.833 atm O D 1.00 atm Unanswered Fill-in–the-blank questions: Complete the following sentences with the correct answer for each blank. Question 32 Fill in the Blanks As the temperature of a
SOLVED: The atmospheric pressure in Denver, CO is 633 mmHg. What is this pressure in atm? ⏩SOLVED:Make the indicated pressure conversions. a. 45.2 kPa to… | Numerade If the atmospheric pressure is 800 mmHg, then what is the pressure of the dry gas you collected? When 0.600 liters Ar at 1.20 atm and 227 C is mixed with 0.200 liters of O2 at 501 torr and 127 C in a 400 ml flask at 27 C, what is the pressure in the flask?